
views
Understanding the Basics

Know what the empirical formula is. In chemistry, the EF is the simplest way to describe a compound—it is basically a list of the elements that make up a compound, organized by percentage. It's important to note that this simple formula does not describe the arrangement of the atoms within the compound, it simply states which elements compound is made of. For example: A compound that is made up of 40.92% Carbon, 4.58% hydrogen, and 54.5% Oxygen would have an empirical formula of C3H4O3 (we will go through an example of how to find the EF of this compound in Part Two).

Understand the term 'percent composition'. 'Percentage composition' refers to the percent of each individual atom in the whole compound that we are looking at. To find the empirical formula of a compound, we must know the percentage composition of the compound. If you are finding the empirical formula for homework, you will most likely be given the percentages. In a chemistry lab, to find the percentage composition, the compound would be examined through some physical experiments and then quantitative analysis. Unless you are in a lab, you will not need to actually do these experiments.

Be aware that you will be dealing with gram atoms. A gram atom is the specific amount of an element that's weight in grams equals its atomic mass. To find a gram atom, the equation is: The percent of the element in the compound (%) divided by the element’s atomic mass. For example, let’s say that we have a compound that is made up of 40.92% carbon. The atomic mass of carbon is 12 so our equation would be 40.92 / 12 = 3.41.

Know how to find the atomic ratio. When you are working with a compound, you will have more than one gram atom to calculate. After you have found all of the gram atoms in your compound, look at all of them. To find the atomic ratio, you will have to pick out the gram atom that is the smallest out of all of the gram atoms that you calculated. You will then divide all of your gram atoms by the smallest gram atom. For example: Let’s say that we are working with a compound that has three gram atoms: 1.5, 2 and 2.5. The smallest gram atom out of those three numbers is 1.5. So to find the atomic ratio, you must divide all of the numbers by 1.5 and then separate them with the symbol for ratio :. 1.5 / 1.5 = 1. 2 / 1.5 = 1.33. 2.5 / 1.5 = 1.66. So your atomic ratio is 1 : 1.33 : 1.66.
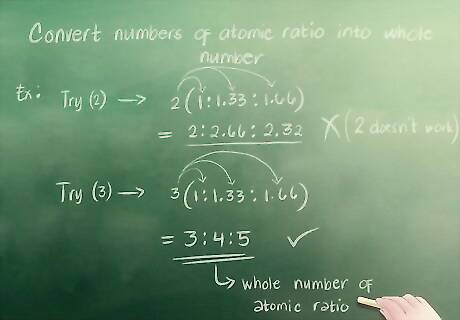
Understand how to convert numbers of atomic ratio into whole numbers. When writing an empirical formula, you need whole numbers. This means you can’t use a number like 1.33. After you have found your atomic ratio, you need to convert any partial numbers (again, like 1.33) into whole numbers (like 3). To do this, you have to find a whole number that can be multiplied by each individual number in your atomic ratio to get a whole number. For example: Try 2. Multiply the numbers in your atomic ratio (1, 1.33, and 1.66) by 2. You get 2, 2.66, and 3.32. These are not whole numbers so 2 doesn’t work. Try 3. You get 3, 4, and 5 when you multiply 1, 1.33, and 1.66 by 3. Therefore, your atomic ratio of whole numbers is 3 : 4 : 5.
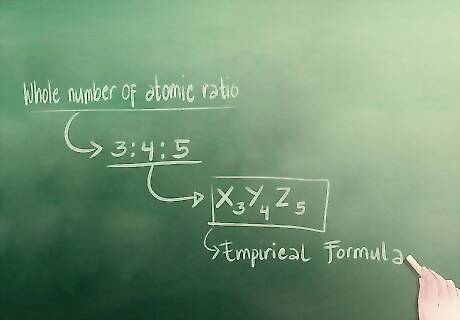
Understand what those whole numbers mean for the empirical formula. The whole number ratio that we just solved actually fits in to the empirical formula. Those three whole numbers are the little numbers that hang at the foot of each letter that represents a separate element of the compound. For example, our made up empirical formula would look like: X3Y4Z5
Finding the Empirical Formula
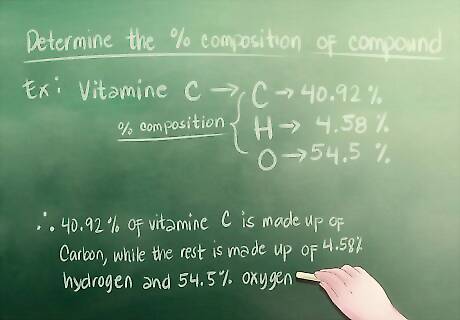
Determine the percentage composition of your compound. If you are trying to find the empirical formula for a homework assignment, you will most likely be given the percentage composition—you just need to know where to look. For example: Let’s say that the assignment asks you to look at a sample of vitamin C. It lists 40.92% Carbon, 4.58% hydrogen 54.5% Oxygen—this is the percent composition. 40.92% of the vitamin C is made up of carbon, while the rest is made up of 4.58% hydrogen and 54.5% oxygen.
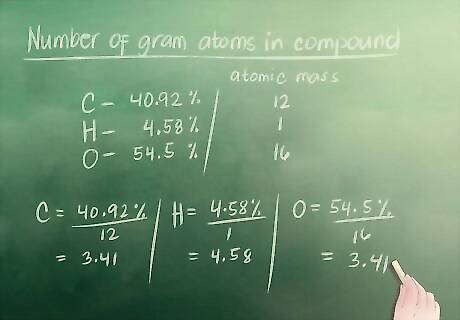
Find the number of gram atoms in the compound. As discussed in Part 1, the equation for finding the number of gram atoms is: The percent of the element in the compound (%) divided by the element’s atomic mass. For our example, the atomic mass of carbon is 12, hydrogen is 1 and oxygen is 16. Number of gram atoms of carbon = 40.92 / 12 = 3.41 Number of gram atoms of hydrogen = 04.58 / 01 = 4.58 Number of gram atoms of oxygen = 54.50 / 16 = 3.41
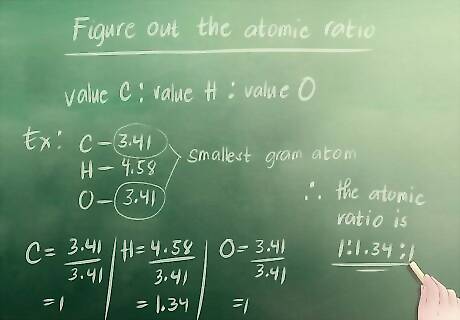
Figure out the atomic ratio. Look for the gram atom that is the smallest of all of the gram atoms that we just calculated. For our example, this is 3.41 (carbon or oxygen—they both have the same value). You must then divide all of the gram atom values by this number. You write the ratio like this: value Carbon : value Hydrogen : value Oxygen. Carbon: 3.41 / 3.41 = 1 Hydrogen: 4.58 / 3.41 = 1.34 Oxygen: 3.41 / 3.41 = 1 The atomic ratio is 1 : 1.34 : 1.
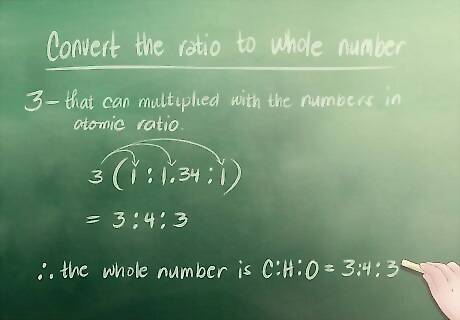
Convert the ratio to whole numbers. If your atomic ratio has whole numbers you can skip this step. For our example, however, we have to convert 1.34 to a whole number. Our smallest whole number that can be multiplied with the numbers in our atomic ratio to create whole numbers is 3. 1 x 3 = 3 (this works because 3 is a whole number). 1.34 x 3 = 4 (4 is also a whole number). 1 x 3 = 3 (again, 3 is a whole number). Our whole number ratio is therefore Carbon(C) : Hydrogen(H) : Oxygen(O) = 3 : 4 : 3
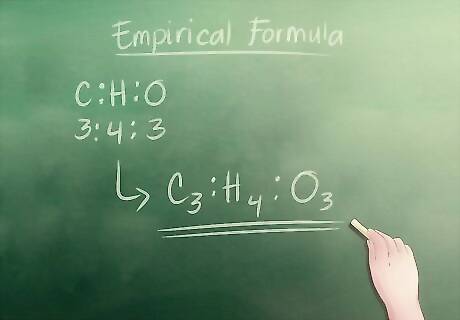
Write down the empirical formula. To do this, all you have to do is write the letters of each component, in this case C for carbon, H for hydrogen, and O for oxygen, with their whole number counter parts as subscripts. The empirical formula for our example is: C3H4O3

















Comments
0 comment