views
X
Research source
Assigning Oxidation Numbers Based on Chemical Rules
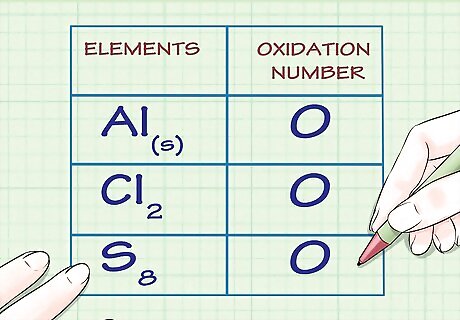
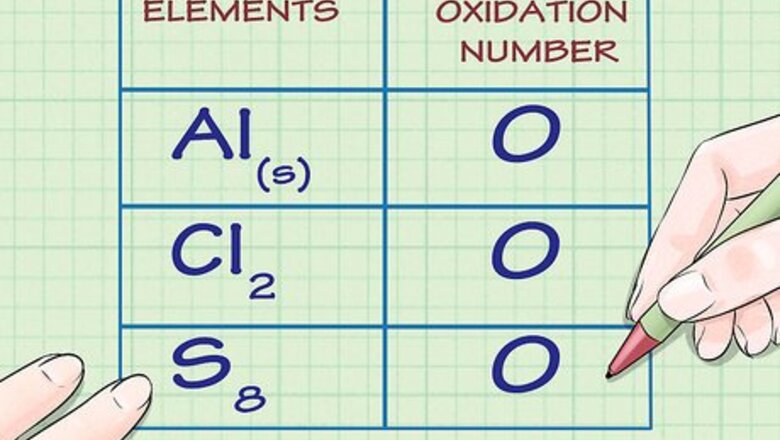
Determine whether the substance in question is elemental. Free, uncombined elemental atoms always have an oxidation number of 0. This is true both for atoms whose elemental form is composed of a lone atom, as well as atoms whose elemental form is diatomic or polyatomic. For example, Al(s) and Cl2 both have oxidation numbers of 0 because they are in their uncombined elemental forms. Note that sulfur's elemental form, S8, or octasulfur, though irregular, also has an oxidation number of 0.
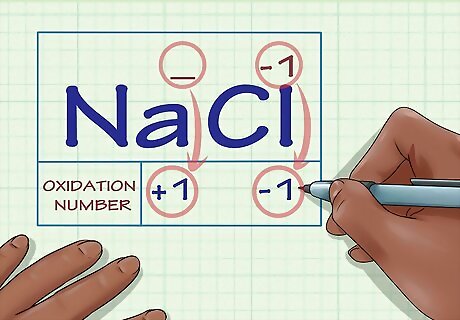
Determine whether the substance in question is an ion. Ions have oxidation numbers equal to their charge. This is true both for ions that are not bound to any other elements as well as for ions that form part of an ionic compound. For instance, the ion Cl has an oxidation number of -1. The Cl ion still has an oxidation number of -1 when it's part of the compound NaCl. Because the Na ion, by definition, has a charge of +1, we know that the Cl ion has a charge of -1, so its oxidation number is still -1.
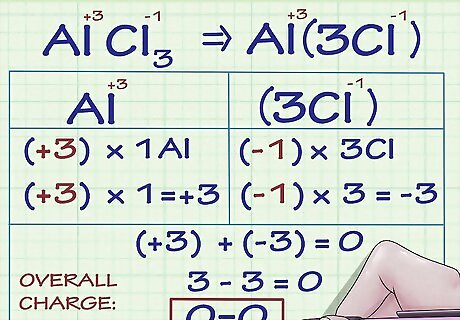
Know that multiple oxidation numbers are possible for metallic ions. Many metallic elements can have more than one charge. For instance, the metal iron (Fe) can be an ion with a charge of either +2 or +3. Metallic ions' charges (and thus oxidation numbers) can be determined either in relation to the charges of other atoms in the compound they are a part of, or, when written in text, by roman numeral notation (as in the sentence, "The iron(III) ion has a charge of +3."). For example, let's examine a compound containing the metallic aluminum ion. The compound AlCl3 has an overall charge of 0. Because we know that Cl ions have a charge of -1 and there are 3 Cl ions in the compound, the Al ion must have a charge of +3 so that the overall charge of all the ions adds to 0. Thus, Al's oxidation number is +3 in this compound.
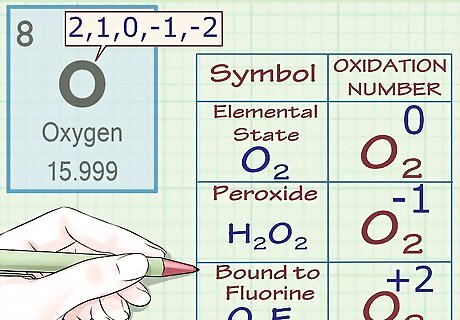
Assign an oxidation number of -2 to oxygen (with exceptions). In almost all cases, oxygen atoms have oxidation numbers of -2. There are a few exceptions to this rule: When oxygen is in its elemental state (O2), its oxidation number is 0, as is the case for all elemental atoms. When oxygen is part of a peroxide, its oxidation number is -1. Peroxides are a class of compounds that contain an oxygen-oxygen single bond (or the peroxide anion O2). For instance, in the molecule H2O2 (hydrogen peroxide), oxygen has an oxidation number (and a charge) of -1. When oxygen is part of a superoxide, its oxidation number is -1⁄2. Superoxides contain the superoxide anion O2. When oxygen is bound to fluorine, its oxidation number is +2. See fluorine rule below for more info. However, there is an exception: in (O2F2), the oxidation number of oxygen is +1.
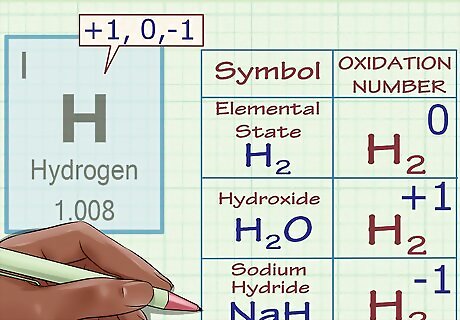
Assign an oxidation number of +1 to hydrogen (with exceptions). Like oxygen, hydrogen's oxidation number is subject to exceptional cases. Generally, hydrogen has an oxidation number of +1 (unless, as above, it's in its elemental form, H2). However, in the case of special compounds called hydrides, hydrogen has an oxidation number of -1. For instance, in H2O, we know that hydrogen has an oxidation number of +1 because oxygen has a charge of -2 and we need two +1 charges to make the compound's charges add up to zero. However, in sodium hydride, NaH, hydrogen has an oxidation number of -1 because the Na ion has a charge of +1 and, for the compound's total charge to equal zero, hydrogen's charge (and thus oxidation number) must equal -1.
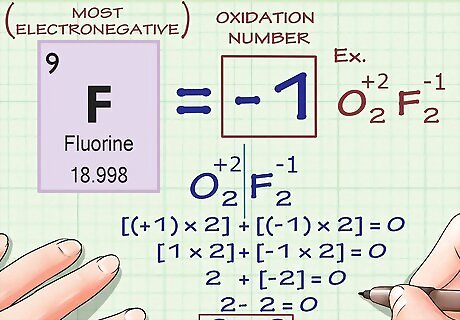
Fluorine always has an oxidation number of -1. As noted above, the oxidation numbers of certain elements can vary for several factors (metal ions, oxygen atoms in peroxides, etc.) Fluorine, however, has an oxidation number of -1, which never changes. This is because fluorine is the most electronegative element - in other words, it is the element least-likely to give up any of its own electrons and most-likely to take another atom's. Therefore, its charge doesn't change.

Set the oxidation numbers in a compound equal to a compound's charge. The oxidation numbers of all the atoms in a compound must add up to the charge of that compound. For example, if a compound has no charge, the oxidation numbers of each of its atoms must add up to zero; if the compound is a polyatomic ion with a charge of -1, the oxidation numbers must add up to -1, etc. This is a good way to check your work - if the oxidation in your compounds don't add up to the charge of your compound, you know that you have assigned one or more incorrectly.
Assigning Numbers to Atoms Without Oxidation Number Rules

Find atoms without oxidation number rules. Some atoms don't have specific rules about the oxidation numbers they can have. If your atom doesn't appear in the rules above and you're unsure what its charge is (for instance, if it's part of a larger compound and thus its individual charge is not shown), you can find the atom's oxidation number by process of elimination. First, you'll determine the oxidation of every other atom in the compound, then you'll simply solve for the unknown based on the overall charge of the compound. For example, in the compound Na2SO4, the charge of sulfur (S) is unknown - it's not in its elemental form, so it's not 0, but that's all we know. This is a good candidate for this method of algebraic oxidation number determination.
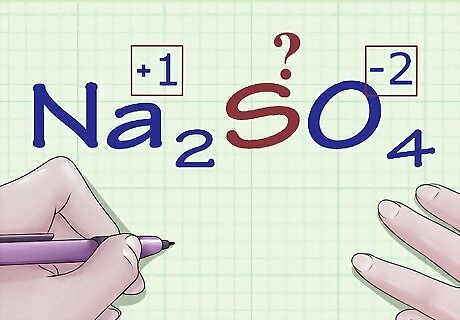
Find the known oxidation number for the other elements in the compound. Using the rules for oxidation number assignment, assign oxidation numbers to the other atoms in the compound. Be on the lookout for any exceptional cases for O, H, etc. In Na2SO4, we know, based on our set of rules, that the Na ion has a charge (and thus oxidation number) of +1 and that the oxygen atoms have oxidation numbers of -2.
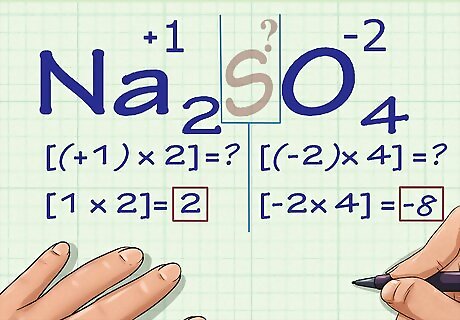
Multiply the number of each atom by its oxidation number. Now that we know the oxidation number of all of our atoms except for the unknown one, we need to account for the fact that some of these atoms may appear more than once. Multiply each atom's numeric coefficient (written in subscript after the atom's chemical symbol in the compound) by its oxidation number. In Na2SO4, we know there are 2 Na atoms and 4 O atoms. We would multiply 2 × +1, the oxidation number of Na, to get an answer of 2, and we would multiply 4 × -2, the oxidation number of O, to get an answer of -8.
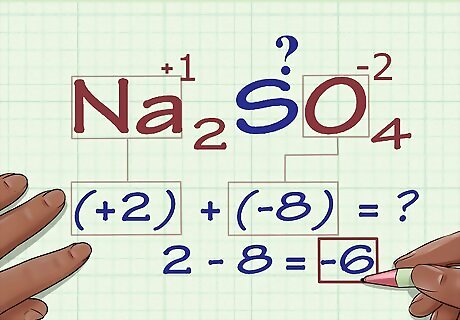
Add the results together. Adding the results of your multiplications together gives the compound's current oxidation number without taking into account the oxidation number of your unknown atom. In our Na2SO4 example, we would add 2 to -8 to get -6.
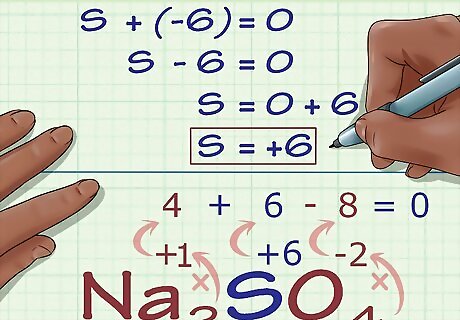
Calculate the unknown oxidation number based on the compound's charge. You now have everything you need to find your unknown oxidation number using simple algebra. Set an equation that has your answer from the previous step plus the unknown oxidation number equal to the compound's overall charge. In other words: (sum of known oxidation numbers) + (unknown oxidation number you are solving for) = (charge of the compound). In our Na2SO4 example, we would solve as follows: (Sum of known oxidation numbers) + (unknown oxidation number you are solving for) = (charge of the compound) -6 + S = 0 S = 0 + 6 S = 6. S has an oxidation number of 6 in Na2SO4.


















Comments
0 comment