views
Performing Fire Tricks Safely

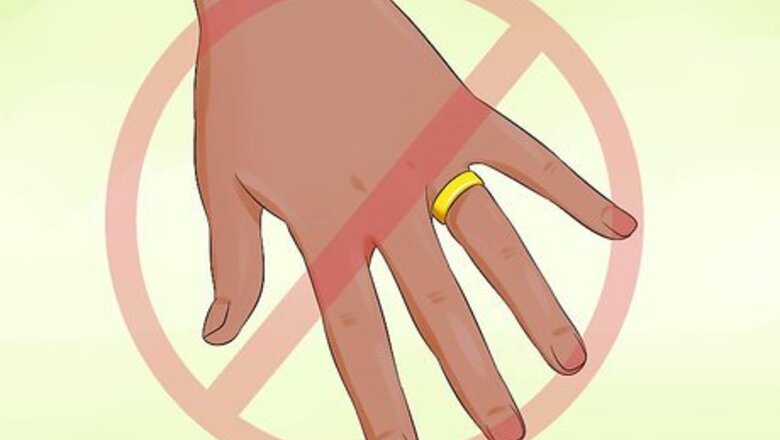
Remove all rings and other jewelry. Before getting started, take off all rings, watches, bracelets, and other accessories and set them aside. These contain metals and other materials that may react unpredictably when exposed to flammable chemicals. And besides, you don’t want to risk ruining them. Your hands should be free and clear while attempting to manipulate flammable compounds.
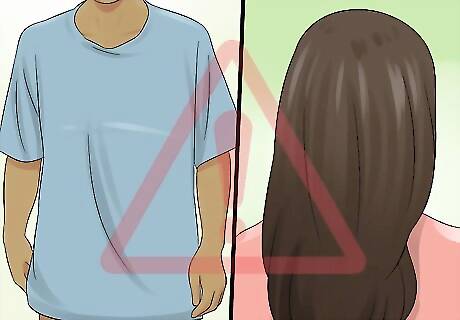
Watch out for loose-fitting clothing and long hair. It’s best to wear short sleeves or snug clothing that rests close to the skin. Roll up long shirt sleeves and be cautious when working with an open flame. Baggy, loose-fitting garments will hang nearer to the fire and flammable solutions, and may get too close for comfort. Long hair should also be pulled back tight or kept under a cap to keep it out of the way. If you have thick facial hair, be sure to keep your face a safe distance away from the flame at all times. In any controlled scenario involving fire, hair and clothing are a possible hazard. Keep the rest of your body a safe distance away from the hand that is holding the fire. Fabrics like cotton, rayon, and acetate catch fire easily and burn quickly.
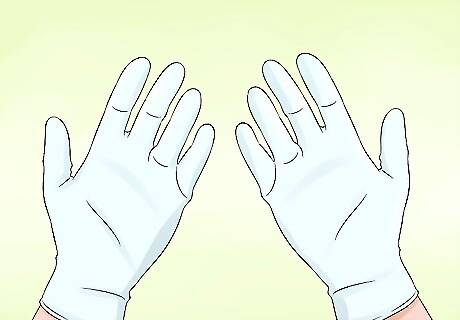
Don’t leave any part of your hand exposed. Submerge your whole hand in the alcohol or gas-infused soap solution to coat the entirety of the outer surface of your skin. Be careful not to leave any exposed spots or allow the skin to dry before lighting it. These types of fire tricks pose a relatively low risk of injury or mishap when executed correctly, but accidents may occur if you’re careless or unprepared. Because of their high degree of flammability, gases like butane and methane burn extremely hot. Injury may result if any part of your skin not covered by the liquid solution is allowed to come into contact with the fire. For maximum protection, consider wearing rubber laboratory gloves when lighting your hands. It’s not quite as daring, but you’ll have a much lower chance of burning yourself.
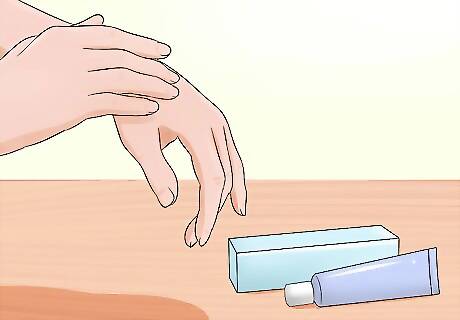
Have safety measures in place. Try out fire experiments by a running sink the first few times around, or keep a bowl or spray bottle of cold water nearby. In the unfortunate event that you do get burned, wash the area thoroughly and apply a burn ointment to soothe the pain. Also, it’s best if there’s another person around when working with fire. If something goes wrong, you’ll want someone else there to help. Keep a fire extinguisher handy in case the flames catch something that they’re not supposed to. Serious burns should be examined and treated by a medical specialist immediately.
Practice on another object first. Try lighting another object first to prevent accidental injury when experimenting with flammable solutions. A scrap piece of wood or a non-combustible material like hard metal or stone can be used as a control until you feel comfortable attempting the feat on yourself. Do not use any object that is flammable or explosive, or that might immediately melt or conduct enough heat to burn you. Almost anything will burn once it has been coated in the alcohol or gas solution. Choose a testing material that won't catch once the solution has burned out, such as metal, rubber, ceramic or fiberglass. Set up the experiment outdoors, or someplace where a fire won't spread if something goes wrong.
Using Alcohol and Water
Wash and dry your hands. Before attempting to set your hands on fire, first give them a good wash and make sure they’re completely dry. Use a mild soap with warm water and scrub your hands together vigorously. The natural oils that build up on your skin can interfere with the chemicals you will be using to protect and light your hands. Do not use hand sanitizer to wash your hands. Most hand sanitizers contain alcohol, which is itself mildly flammable.

Mix together equal parts water and rubbing alcohol. Pour around 10 ounces of water into an open container of medium size. Then, add an equal amount of isopropyl alcohol (ordinary rubbing alcohol). You want to aim for roughly an even mixture of alcohol and water. Whisk the alcohol and water together lightly to mix them. Some bottled isopropyl alcohol comes already diluted. Consider this when mixing the alcohol with water. If it’s a strong alcohol, like a 90/10 dilution, use about 11 ounces with 9 ounces of water. For weaker kinds, like a 70/30 dilution, you’ll need to use significantly more alcohol, closer to 14 ounces, with 6 ounces of water.

Soak your hands in the alcohol solution. Place one or both hands in the alcohol solution. Let them soak for up to a minute. Isopropyl alcohol is mildly flammable, but diluting it with water and soaking your hands will protect you from the fire while the alcohol vapors burn themselves out. Make sure you completely submerge your hand so that the flame will burn evenly. The longer you soak your hands, the more water absorbs into your skin, saturating it and safeguarding you from burns.
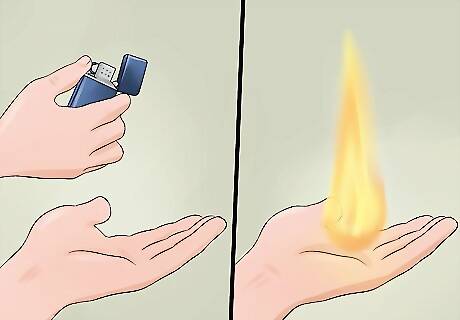
Ignite the alcohol solution on your hands. With your hand still wet with the solution, use a lighter with a long stem to ignite the fire. If you’ve soaked both hands, have a friend help you. When lit, the alcohol solution will produce a quick burst of flame, leaving your hands untouched. As long as your hand is thoroughly wet with the solution, the fire won’t burn you. Alcohol doesn’t burn particularly long or hot, so this version, while providing the safest conditions, won’t be the most impressive. Wash your hands again when you’re done to remove any remaining traces of alcohol.
Using Flammable Gas and Soapy Water
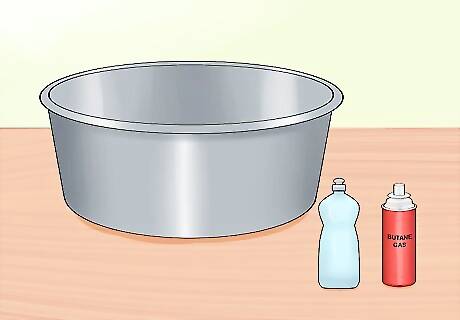
Gather your materials. Get together everything you need to create a fireball using soapy water and flammable gas. For this trick, you’ll need a large, open container, water, liquid detergent and access to a valve or canister of flammable gas like butane or methane. You may also need a rubber hose to direct the flow of gas into the soap solution. Flammable gases, especially in compressed canisters, should only be used under the supervision of an expert or knowledgeable adult. Butane can be purchased in small bottles with built-in nozzles for simple culinary use.

Combine the soap and water in large container. Fill your container about ¾ with cold water. Squeeze in 1-2 ounces of liquid soap and stir until the soap is dissolved in the water. You don’t need to use a lot of soap—just enough to create a mild solution. The soap and water will form a protective layer on your skin to keep you from being burned. Any regular liquid dish soap will do the trick. Stay away from hand soaps and liquid laundry detergents. The lipids in the soap solution will naturally separate from the gas bubbles, keeping them away from your skin.

Add the flammable gas to the soap solution. Begin infusing the gas into the soap solution. If you’re using a commercial butane canister, simply place the nozzle below the surface of the water and give it a few squeezes. If you’re using a large methane tank or gas valve, release the gas slowly into the soap solution until it begins to bubble. Gases like butane and methane are lighter than air, so the bubbles will continue to rise and grow larger the more gas you add. The bubbles themselves will be extremely flammable, so be careful and only use a little at a time. Methane bubbles, in particular, are light enough to stack up on top of one another interminably until the gas supply is shut off.

Coat your hands completely in the solution. Dip your hand into the gas-infused soap solution. Coat your entire hand to make sure the solution sticks to your skin. Most of the gas will be trapped in the bubbles, so scoop up a handful for a bigger flame that burns longer. Whatever gas bubbles come into contact with your hands will burn out before they reach your skin through the soap solution.

Light your hands. Take a lighter to the gas bubbles and set them ablaze. Both butane and methane are extremely flammable, so watch out! The fire will burn intensely for a few seconds, but there’s no need to worry. The soapy water solution will act as a barrier between the flame and your skin. The bubbles and fumes from the gas will continue rising even after they’ve touched your skin. This means that they’ll catch fire as they’re moving away from you, making the experiment safe. Look out for drips and drifting bubbles. These can be ignited on their own!




















Comments
0 comment