views
The government on Thursday issued a regulatory order saying that India’s drug regulator will take decision on applications seeking approval for restricted emergency use of foreign produced vaccines within three working days from date of submission. The move comes at a time when India is faced with an alarming surge in coronavirus cases.
Several experts have reiterated the need to ramp up the vaccination drive to take control of the coronavirus situation which has put India’s healthcare system under pressure.
While India currently has two vaccines, Serum Institute of India’s Covishield and Bharat Biotech’s Covaxin, being administered, the shortage of jabs has overwhelmed the healthcare system further. The Central Drugs Standards Control Organisation (CDSCO) headed by Drugs Controller General of India (DCGI) has said that it has prepared detailed guidelines specifying regulatory pathway for the approval of foreign-approved Covid-19 vaccines based on NEGVAC recommendations, according to a ANI report.
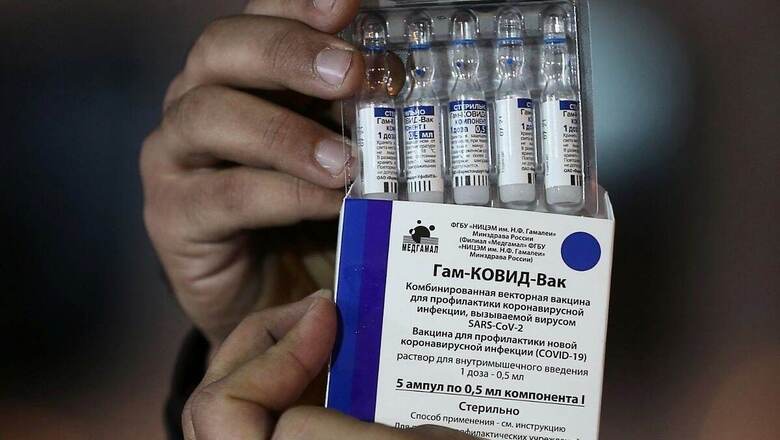
Earlier this week, Russian vaccine Sputnik V received a nod from India’s expert panel for emergency use. Dr Reddy’s Laboratories will start importing bulk doses of its anti-coronavirus vaccine Sputnik V from Russia between mid-April to June. According to a contract signed between Hyderabad-based Dr Reddy’s Laboratories (DRL) and Russian Direct Investment Fund (RDIF), India will receive about 250 million doses of Sputnik V that would be sufficient for 125 million people.
Deepak Sapra, DRL director and CEO-API and services, said the company has the capacity to procure more number of doses depending on domestic requirement and mutual consent with RDIF. Once the fresh batch of Sputnik vaccines arrive in India, Dr Reddy’s said 60-70 per cent of the shots will be made domestically.
Other vaccines in the pipeline for emergency use in India are Johnson &Johnson, Pfizer, Moderna, Zydus Cadila and Novavax.
The health ministry had earlier said it would expedite the approval of vaccines not made in India to “expand the basket of vaccines for domestic use and hasten the pace and coverage of vaccination”. The jabs will need to have been already been granted emergency-use authorisation by regulators, such as the US Food and Drug Administration, the European Medicine Agency and others in Britain and Japan or the World Health Organisation, the ministry said. A requirement for pre-approval clinical trials would be replaced by post-authorisation trials.
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.




















Comments
0 comment