views
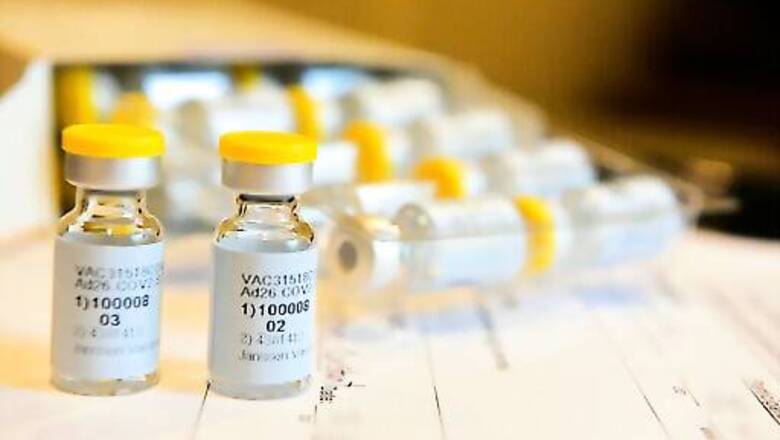
The US is looking at joint production of Johnson and Johnson’s Covid-19 vaccine in India and ways to help manufacturers like the Serum Institute of India (SII) to boost production, Daniel B Smith, the Charge D’Affaires of the US embassy, said on Tuesday.
Smith also said that the efficacy of AstraZeneca’s Covid-19 vaccine manufactured at a production facility in Baltimore is not yet clear and the Food and Drug Administration has not yet certified that the doses are available for anyone’s use or for export.
Here is how the vaccine may add ammo to India’s fight against the pandemic:
Who should get vaccinated?
The J&J vaccine is recommended for people aged 18 years and older.
Who should not get vaccinated?
If you have had a severe allergic reaction (anaphylaxis) or an immediate allergic reaction—even if it was not severe—to any ingredient in the J&J COVID-19 vaccine (such as polysorbate), you should not take the shot.
An allergic reaction is considered severe when a person needs to be treated with epinephrine or if they must go to the hospital. Experts refer to severe allergic reactions as anaphylaxis.
An immediate allergic reaction means a reaction within 4 hours of getting vaccinated, including symptoms such as hives, swelling, or wheezing (respiratory distress).
What are the possible side effects of the jab?
Pain in the arm, redness or swelling. Tiredness throughout the rest of your body, headache, muscle pain, chills, fever and nausea.
These side effects usually start within a day or two of getting the vaccine. Side effects might affect your ability to do daily activities, but they should go away in a few days.
Is there fainting recorded after the vaccine?
Fainting (syncope) and other events that may be related to anxiety like rapid breathing, low blood pressure, numbness, or tingling can happen after getting any vaccine. Although uncommon, these events are not unexpected, and they are generally not serious.
According to information from the Vaccine Adverse Event Reporting System (VAERS),there have been 653 reports of fainting events(fainting and near-fainting) among nearly 8 million doses of J&J/Janssen vaccine administered in the United States in March and April 2021. This translates to a rate of about 8 fainting events for every 100,000 doses of the J&J vaccine given.
What were the clinical trial results?
In clinical trials, side effects were common within 7 days of getting vaccinated but were mostly mild to moderate. Side effects were more common in people 18–59 years old compared to people 60 years and older.
How well does the vaccine work?
The J&J vaccine was 66.3% effective in clinical trials (efficacy) at preventing laboratory-confirmed COVID-19 illness in people who had no evidence of prior infection 2 weeks after receiving the vaccine. People had the most protection 2 weeks after getting vaccinated.
The vaccine had high efficacy at preventing hospitalization and death in people who did get sick. No one who got COVID-19 at least 4 weeks after receiving the J&J vaccine had to be hospitalized.
Early evidence suggests that the J&J vaccine might provide protection against asymptomatic infection, which is when a person is infected by the virus that causes COVID-19 but does not get sick.
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.













Comments
0 comment