views
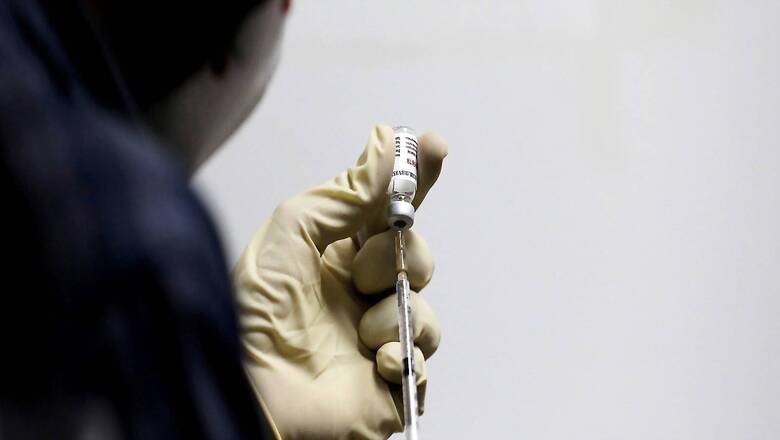
India has begun choosing Covid-19 patients ahead of the rollout of the second phase of World Health Organization’s Solidarity trial to check the efficacy of two drugs, country’s chief trial coordinator Sheela Godbole told News18.com.
The Solidarity trial, an international clinical trial meant to help find an effective treatment for Covid-19, compares options against the standard of care and assesses its relative effectiveness against the disease.
By enrolling patients in multiple countries, the trial aims to rapidly discover whether any of the drug slows disease progression or improves survival. Last year, it tested Remdesivir, Hydroxychloroquine, Lopinavir and Interferon.
“The Solidarity Trial in 2021 is essentially a continuation of the previous trial.
As before the design is an adaptive, randomized controlled platform trial being conducted among hospitalized patients,” said Dr Godbole, who is also the scientist at ICMR-National AIDS Research Institute (NARI) in Pune.
“There is no cap on how many patients may be recruited by any one site or country. We will try to recruit as many as possible in India.”
In India, the primary objective of the trial is to understand and provide reliable estimates about the efficacy of two study drugs — Imatinib and Infliximab – on preventing mortality in patients hospitalised for Covid-19.
Three arms would test anti-cancer drug Imatinib and biological drug Infliximab apart from patients under the arm called local standard of care.
The secondary objective of the trial is to “assess the impact of the drugs on initiation of ventilation and duration of hospital stay.”
In India, randomising of the patients has already begun — a process on the basis of which patients are assigned one of the any three arms in the trial.
Design of the Trial
Under the trial, one arm will be administered 400 milligrams of Imatinib once daily for 14 days along with local standard of care.
Another arm will be given Infliximab injection, which is otherwise used in chronic conditions such as rheumatoid arthritis, Crohn’s disease, Psoriasis, Inflammatory Bowel disease. “In the trial it will be given as a single dose (5 mg per kilogram per dose) single IV over 2 hours along with local standard of care.
Third arm will only consist of a local standard of care, which means symptoms based treatment.
Why are These Two Drugs Chosen?
Dr Godbole said Imatinib is a tyrosine kinase inhibitor used to treat leukemia, myelodysplastic/ myeloproliferative diseases, hypereosinophilic syndrome, gastrointestinal stromal tumors.”
“This is expected to have a role in reversing pulmonary capillary leak and preventing lung injury,” she said while further explaining that “it will target both the coronavirus and inflammation – blocking viral infiltration of human cells and reducing the activity of pro-inflammatory proteins, cytokines.”
Infliximab, which is a monoclonal anti-tumor necrosis factor-alpha antibody used to treat autoimmune conditions such as rheumatoid arthritis, Crohn’s disease, psoriasis and inflammatory bowel disease.
“Many cytokines are elevated in Covid-19 disease, including TNF (TNF is a pivotal cytokine that controls the inflammatory cascades – involved in a broad spectrum of inflammation). Anti-TNF drug can treat progressive disease in addition to offering prophylaxis,” she said.
Will Fall in Cases Delay Trials in India?
The fall in the number of cases in India is good news, she said.
“The most important feature of the Solidarity Trial is that it’s a multi-country trial with over 50 countries who are interested in participation. More and more countries have begun recruitment. Some countries may have more cases than others over time.”
She further said, “What is important is that together globally we recruit adequate numbers to find answers to our research questions.”
In any pandemic, Dr Godbole said, where there is waxing and waning in burden of infections, it is expected that some countries would not recruit as many cases.
“Thus, at this time, we don’t see any major threat to the trial.”
Read all the Latest India News here
















Comments
0 comment