views
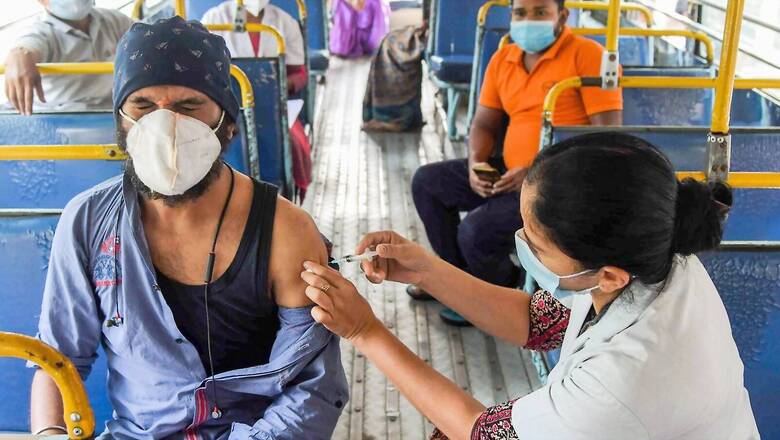
India is ending 2021 with hectic developments in the fight against Covid-19, heading into the new year with an expanded vaccination drive to cover teenagers, introduce precautionary shots for vulnerable population and more vaccines in its arsenal.
Amid surging cases of Omicron, the push came from Prime Minister Narendra Modi’s surprise address to the nation on December 25 when he announced that children aged between 15 and 18 would start getting Covid-19 vaccines from January 3. Amid demand for boosters, Modi announced that healthcare and frontline workers and senior citizens with comorbidities would get what he called ‘precautionary shots’.
In the days that followed, officials running the vaccination drive and experts weighed in on burning questions like the difference between precautionary dose and booster dose, which vaccine will be administered to teens, will India go in for mix-and-match approach and what more is in the pipeline.
Here are 10 exclusive stories from News18.com’s extensive coverage that answer these and more:
The Year Ahead
First thing’s first: will 2022 be better? Like most things with the pandemic, it depends. Experts say individual behavior will remain a major factor despite the ramped up vaccination drive but add that our bodies are learning. Vaccine expert and scientist Dr Gagandeep Kang uses the analogy of a newborn baby to explain how, at one point, Covid-19 may become as casual as “cold and flu”.
We know better and more than we did in 2020 and have made giant strides in vaccine development. While this can help us tame the virus, we may not be able to completely trounce it. For now, scientists predict an obvious race of vaccines and variants in the coming years till the coronavirus reaches endemicity. READ FULL STORY HERE
Modi’s Surprise Announcement
PM’s announcement on Christmas took many by surprise specially as healthcare and vaccine officials had been saying there was no urgency in launching vaccine for minors and boosters for adults.
The announcement was, however, preceded by several factors, the biggest being the surge in Omicron variant cases in states like Maharashtra and Delhi that were hit hard by the Delta wave in April. India has so far reported 653 Omicron cases across 21 states and Union Territories. Another factor was the concern over potential drop in immunity levels of those who got inoculated right at the beginning of the vaccine drive in January.
It is also hoped that the second phase of vaccination will push those yet to take their first or second dose to get vaccinated. READ FULL STORY HERE
Vaccination 2.0: Who’s In?
As per 2011 Census projections, the next phase of the Covid-19 vaccination drive will potentially include 20.4 crore beneficiaries. While the number of teenagers aged 15-18 is estimated at 7.4 crore, around 3 crore healthcare and frontline workers could queue up for the precautionary dose. The number of people aged over 60 with comorbidities is estimated to be 10 crore.
However, the final figure of immediate beneficiaries for the precautionary dose depends on the gap to their last dose. For the senior citizens, it also depends on whether their ailment falls under 20 comorbidities listed by healthcare authorities. READ FULL STORY HERE
Which Comorbidities are Covered?
The government will allow precautionary doses on the basis of 20 listed comorbidities: cardiovascular diseases, diabetes, stem cell transplant, kidney disease or on dialysis, cirrhosis, cancer, sickle cell disease, and current prolonged use of steroids or immunosuppressant drugs.
While senior citizens will not have to produce a medical certificate or prescription or proof of their condition, they are suggested to go for the precautionary dose only after they are medically advised to do so. READ FULL STORY HERE
Why Precautionary and Not Booster?
This was the point of curiosity in PM Modi’s announcement. What is precautionary dose and how is it different from booster dose? While initially it was presumed that both mean the same, top officials in the Union Health Ministry told News18.com that precautionary dose is different from booster dose.
An official said it has been termed ‘precautionary dose’ since part of the target population is elderly and their bodies might not have generated the immune response with first two doses as much as young or healthy bodies might have. “Hence, the third dose is a matter of precaution because a resurgence of a new variant has been announced,” the official said.
Sources also revealed that India might not go in for mix-and-match vaccinations, meaning the third dose will likely be the same vaccine administered during first two doses. READ FULL STORY HERE
Only Covaxin for Teens Right Now
For now, Bharat Biotech’s Covaxin will be the only shot available to children aged 15 to 18 when inoculation begins next month. Zydus Cadila’s ZyCoV-D, touted as the world’s first DNA vaccine, was earlier said to be in the running but was missing from the guidelines issued by the Health Ministry on Monday.
CoWIN chief RS Sharma has said that eligible teens can register on the platform using their school ID card as proof of age since some of them may not yet possess Aadhaar cards. The beneficiaries (aged 15-18 years) can register themselves online through an existing account on Co-WIN or by creating a new account through a unique mobile number. READ FULL STORY HERE
What Happens to ZyCoV-D Now?
Zydus Cadila’s nasal vaccine is not out of the picture yet. Sources told News18.com that the government may decide to extend the expiry date or shelf-life of ZyCoV-D by three more months. The company has already got a stock of 2.37 lakh doses cleared by the country’s apex laboratory, the Central Drugs Laboratory (CDL).
Officials said the vaccine will remain “absolutely safe” after extension of shelf life since requisite studies have already been conducted. “Stability studies were performed on three batches that had shown that the expiry date can easily be pushed from six months to nine months. The vaccine remains extremely safe,” an official said. READ FULL STORY HERE
Will We Get Conventional Boosters?
Sources have told News18.com that separate deliberations are underway on booster shots. The Centre had earlier launched a study to assess the need of booster doses in India, covering 3,000 participants who had received the second dose of Covid-19 vaccine six months ago.
Hyderabad-based Bharat Biotech has also sought permission from India’s drug regulator to conduct clinical trials to evaluate the efficacy of a third dose of Covaxin. Trials have been proposed for 5,000 health volunteers with a gap of six months between second and third dose. It has also proposed trials with 500 HIV-positive patients to gauge efficacy in immunocompromised individuals.
Adar Poonawalla’s Serum Institute of India also sought the approval of Drug Controller General of India (DCGI) to administer Covishield as a booster dose citing adequate stock and a demand for a booster shot, but the request was denied. READ FULL STORY HERE
2 More Vaccines and a Pill on the Way
Serum Institute of India’s Covid-19 vaccine Covovax and Biological E’s vaccine Corbevax may join the list of vaccines in India soon with the country’s central drug authority recommending emergency use authorisation to the two.
The Subject Expert Committee (SEC) of the Central Drugs Standard Control Organisation (CDSCO) has also recommended granting permission to manufacture and market anti-Covid pill Molnupiravir for restricted emergency use. It could conditionally be used to treat adult patients with 93% SpO2 and who have high risk of progression of the disease, including hospitalisation or death.
All recommendations have been sent to the Drugs Controller General of India (DCGI) for final approval. If approved, the number of vaccines with emergency use authorisation will increase to eight. READ FULL STORY HERE
mRNA Vaccine Coming Too?
mRNA technology involves injecting a small part of the virus’s genetic code (RNA) to stimulate the recipient’s immune response but no actual virus is contained in the vaccines. According to Dr Gagandeep Kang, studies have shown it to be the best booster dose. So far, top mRNA vaccines available across countries are Pfizer-BioNtech and Moderna’s shots but Pune-based Gennova Biopharmaceuticals is also developing one.
Sources have told News18.com that Gennova is in talks with the government for its mRNA to be considered for booster shots. Its vaccine has currently progressed to third phase trials.
While available mRNA vaccines require ultra-cold storage of around -70 degree Celsius, Gennova’s technology is much easier to store and transport as it requires temperature of around 2-8 degree Celsius — the usual temperature that every other vaccine would need. READ FULL STORY HERE
Read all the Latest India News here

















Comments
0 comment