views
A vaccination programme that covers a population as vast as India’s 1.3 billion is expected to be a herculean task, but India has implemented national immunisation campaigns with relative success in the past. The need for a faster rollout today—amidst a raging, treacherous second wave of the COVID-19 pandemic—creates its own, largely unprecedented challenges.
The availability of digital platforms can assist in managing and calibrating the vaccine deployment, provided that policies are data-driven and inclusive. In recent days, the central government has been more open about using and sharing data pertaining to vaccine procurements, allowing for evidence-based and faster planning. Will India see better days ahead in its vaccine rollout? This report explains the gargantuan challenges in India’s COVID-19 vaccination campaign. It outlines specific urgent steps that need to be taken if India is to meet its targets.
Launching the Vaccination Campaign
On 19 April 2021, the Press Information Bureau (PIB) made public the details of India’s COVID-19 Vaccination Strategy, which focused on the scale and speed for the third phase. The National Expert Group on Vaccine Administration for COVID-19 (NEGVAC), chaired by Dr V K Paul, Member of the NITI Aayog, had played a key role in designing the strategy. The first phase of India’s COVID-19 mass inoculation programme was launched on 16 January, covering an estimated 30 million healthcare and front-line workers. On the first of March, the country initiated the second phase, aiming to cover people above-45 with co-morbidities and the cohort above 60. This was expanded on 1 April to cover everyone above 45 years, bringing the total to over 300 million people. While this number accounted for only 22 per cent of the population, they were also the high-risk and most vulnerable, accounting for 80 per cent of recorded Covid mortality in the country. The expected timeframe for completing the vaccination drive was six months from the launch.
Out of a target of 600 million doses by end-July, 123.8 million doses had been administered as of 18 April. However, it was also in April when the country suffered the onslaught of a massive second wave. At the peak of the first wave in 2020 in mid-September, India registered 96,000 new cases of infection daily; by 18 April, the daily cases had surpassed 275,000, reaching an all-time high of over 400,000 cases in early May. Daily deaths recorded a steep curve too, crossing 4,500 briefly, and has remained high since.
The sudden rise in cases and deaths compelled the Indian government to announce the third phase of its vaccination strategy in April—to include those above 18 years beginning on 1 May, adding another 600 million people to the list. While vaccines for those above 45 will continue to be free of cost, they could either be free or priced for the 18-45 years’ cohort, as determined by the state governments. The two approved vaccine producers—Serum Institute of India (SII), producing Covishield; and Bharat Biotech (BB), producing Covaxin—would supply 50 per cent of their monthly production from 1 May to the central government. The balance was earmarked for state governments and private hospitals. Both aspects of the decision—opening up to the 18-45 cohort, and dual pricing—were justified as introducing decentralisation and flexibility.
The Demand-Supply Mismatch
Predictably, as cases and deaths rose, the opening up of the vaccination drive led to chaos: the initial lukewarm response to the vaccine rollout gave way to scenes of large crowds in vaccination centres, with many going back home without receiving a shot due to shortages. What ensued was a blame game between the Centre and the states on the one hand, and the vaccine manufacturers, on the other. Analysts have sought to piece together their explanations about national procurement, and availability and production capabilities, in an effort to understand India’s vaccine situation. Data has not been easily available, however, until recently. A number of states have issued global tenders even as they halted the vaccination drives for 18-45-year-old citizens due to lack of supplies, but the response has not been reassuring.
The new disclosure of India’s procurement approach has helped create a better understanding of the bigger picture. Table 1 summarises the procurement details and purchase price of vaccines acquired by the central government, as per publicly available sources.
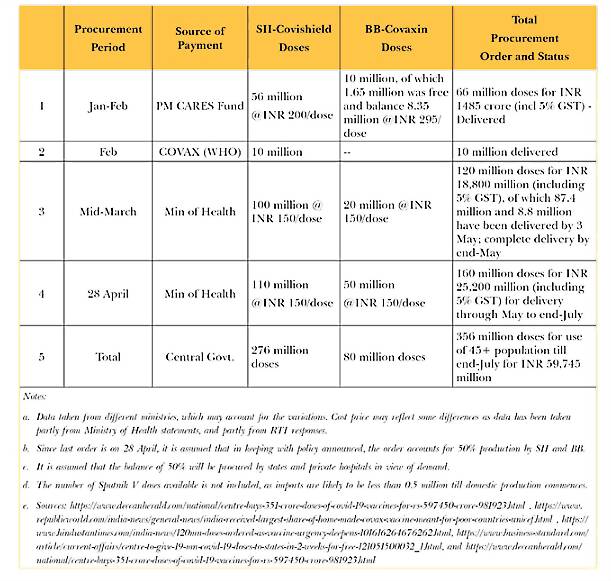
According to a statement by Finance Secretary T.V. Somanathan, the April 2021 procurement was 55 million doses from Bharat Biotech, which is 5 million doses more than what was announced by the Ministry of Health; it is presumed that this difference may have been for forward deployed troops. Since central government procurement has been restricted to 50 percent since May, it can be assumed that 110 million doses of Covishield and 50 million doses of Covaxin can be purchased by the states and private hospitals directly between May and July, bringing the total availability till end-July to 516 million doses—i.e., 356 million with the Centre, and 160 million with states and private hospitals. However, it is unlikely that BB will have 50 million doses to spare after fulfilling supply commitments to the Centre, given its current manufacturing capacity.
The mismatch between supply and demand would explain why the daily vaccination rate dropped from an average of 3.6 million in April to 1.6 million in May, even though the vaccination coverage was expanded to cover people in the 18-45 years’ age group. A total of 200.1 million vaccination doses have been administered as of 27 May. This would mean that if production schedules are able to keep pace and if the balance of 316 million doses are delivered, the daily vaccination rate over the next two months could be 5 million jabs, assuming zero exports. By itself, this number is not difficult to achieve if supplies are secured; in early April, there were days when daily vaccinations crossed 4 million. A nationwide infrastructure of 50,000 centres was set up where each centre can administer 100 vaccine shots daily.
However, due to India’s present situation where daily fatalities are too high and a quick rollout of vaccination is an urgent requirement, the targets are too modest, even to prepare for a looming third wave. At a rate of 1.8 million doses a day, it will take India 2.8 years to inoculate 75 per cent of its population. Increasing the average to 5 million daily, it would still take nearly a year for India to vaccinate the above-18 population—a timespan that is far too long, given the death and disruption that the pandemic and the response to it is causing. A quick vaccine rollout is the country’s only way through. In a clear sign of acceleration, the government of India informed the public on 30 May that while a little more than 79 million doses of COVID-19 vaccine were available with the states in May, nearly 120 million doses will be made available in June. The May-June combined availability of 200 million doses also suggests that any raw material shortages may have been significantly resolved.
Reports indicate that bureaucratic delay has cost India’s vaccine manufacturing expansion plans severely, and there is a growing consensus today that expansion needs to be immediate. Moreover, procurement efforts of vaccines from the international market have to be fast-tracked to help overcome this public health crisis. Increasing the number of vaccination centres is important. However, considering India’s limited resources, including of health workers, what will be more useful is the optimal use of targeted vaccination drives in high population-density areas; as well as drive-in vaccination camps that will be announced in advance. Local community networks should also be used, and the corporate sector could be engaged to take on the responsibility of vaccinating their employees and their dependents.
To address any future mismatch between demand and supply, it is necessary to look at the supply side. Again some clarity has emerged with newly released data. The SII had an initial capacity of about 50 million doses a month, along with a stockpile of 50 million doses in January. As the advance orders were slow in coming, production in February dropped before picking up in March; it currently averages 65 million doses a month. BB too, has a similar story: it had a monthly production of over 5 million doses in January, along with a stockpile of 20 million. Once the Bengaluru facility came online, the production shot up, and is now at 20 million doses. In addition to the 194 million doses supplied to the Centre to date (supplies of the order placed in end-April will continue till end-July), 66 million doses were exported.
In the third week of April, the Centre finally agreed to provide much-needed financial support to SII and BB to enable them to ramp up production. Advances of Rs 30,000 million and Rs 15,000 million, respectively, were announced for the two companies. In addition, three public sector undertakings—Haffkine Institute, Bharat Biologicals and Immunologicals (BIBCOL), and Indian Immunologicals (IIL)—have been provided Rs 1,550 million by the Centre to upgrade facilities and undertake production of at least 30 million Covaxin doses per month (20 million doses for Haffkine Insitute and 10 million doses for BIBCOL; IIL’s quantity has not been made public at the time of writing). Haffkine Institute is unlikely to come on line this year, though the other two may be ready by September. Additionally, the Gujarat government, along with Hester Biosciences and OmniBRx, is also in discussion with Bharat Biotech to scale its manufacturing technology to produce a minimum of 20 million doses of Covaxin, monthly.
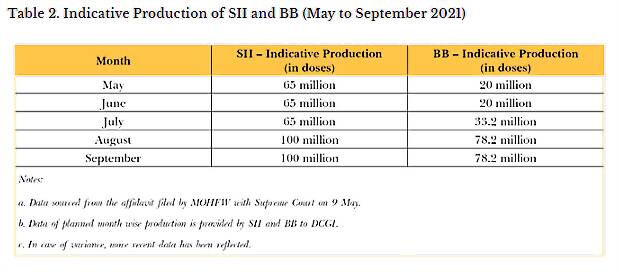
On 9 May, the Centre filed an affidavit with the Supreme Court indicating that during the month, SII and BB were aiming to produce 65 million and 20 million doses, respectively. The following week, SII and BB also filed monthly production plans for the period of June-September with the Drug Controller General of India. However, these monthly supply figures currently do not support a target of 10 million daily vaccinations. Additional options need to be actively explored, in view of raw material shortages reportedly affecting current manufacturing plans. Table 2 shows the monthly production figures for SII and BB.
The Way Forward
Amidst a shortage of vaccine doses in the country, NEGVAC announced that it is expecting roughly 2.16 billion COVID-19 vaccine doses between August and December 2021, all from domestic production, by adding on Sputnik production and other vaccines under development. It is a highly ambitious goal, and the past record of NEGVAC has shown a tendency to overestimate capabilities. It is possible that the expected import of 250 million Sputnik V doses will compensate to some extent for any such overestimation.
It is also important to consider that publicly available production estimates can be misleading since production figures of vaccines that are still under development (numbering around 710 million doses) have been added. Though some of these vaccines are in Phase III trials, many have barely commenced Phase I and Phase II. Biological E and Zydus Cadilla vaccines are undergoing Phase III trials, while BB’s nasal vaccine and Gennova’s mRNA-based vaccine are in Phase I-II trials. Covovax, the Indian variant of the Novavax vaccine, is currently being manufactured by SII—at its own risk before approvals are acquired—and trials are ongoing in India. Monthly production rates of SII and BB are pegged at 150 million and 110 million, respectively—numbers that are inconsistent with what the companies have reported to the government. This is not to suggest that SII and BB cannot expand production, but that proper planning and resource allocation are needed. The expansion approved in April will materialise after three months, along with an established production line.
According to the Centre’s affidavit submitted to the Supreme Court, no government funds were given for vaccine development to SII and BB; rather, the Indian Council of Medical Research (ICMR) provided Rs 350 million to BB for Phase III trials and Rs 110 million to SII for bridging trials. Despite being the first vaccine manufacturer to apply for approval in India, Pfizer later withdrew its application, citing procedural problems; at the same time, the Sputnik V approval application was delayed for weeks until it got fast-tracked during the initial onslaught of the second wave. Although the approval process was not as nimble as what would be ideal during an emergency, it remains unclear whether approving vaccines manufactured by Pfizer or Moderna would have changed the reality in India, given the global shortages where even the wealthiest countries are still standing in line despite their purchasing power.
A recent analysis of company filings by the top four global vaccine manufacturers found that 90 per cent of all the vaccines produced in the first quarter of 2021 in the United States (US) and Europe were administered on populations within North America and Europe. Even in the second quarter, the situation is going to be the same. This brings to the forefront the importance of creating and expanding vaccine production capacity in the developing world, and perhaps explains why India focused on leveraging its own manufacturing capacity rather than betting on the market where too many dollars were chasing too few doses. Unfortunately, India did not prioritise domestic development or production of vaccines in a timely manner, and today the goal of 2.16 billion doses between August and December seems highly ambitious.
At present, there are no advance procurement negotiations beyond July. One of the most successful COVID-19 vaccine programmes in the world, the vaccine deployment strategy of the European Commission, is dependent on two key pillars: securing sufficient production of vaccines for all EU member states through advance purchase agreements, and adapting the regulatory framework for the development and availability of vaccines to meet the urgent requirements of the pandemic. By January 2021, the European Union (EU) had secured more than 2.3 billion doses of vaccines, enough to cover the entire population of the region. It showed the benefits of concluding long-term advance procurement contracts even before the vaccines had received formal approvals. Although India held briefly the record for being the only country to have administered four million jabs in a day—in the first week of April, before China soon overtook it—that is of little use if it fails to address the crisis.
To be sure, the demand for vaccine given India’s population is neither a surprise nor a revelation. Even the original rollout plan placed the target at 600 million doses in six months to 300 million people above 45 years. However, current procurement by the central government is only at 356 million. While it is estimated, based on production levels, that the states and private hospitals will have access to up to 120-160 million doses, a large part of this will be used for the 18-45 age group.
India’s lack of planning became fodder for global discussion due to the sheer scale of the second wave that hit the country. Although the first signs of this wave were already seen in early March, at the time, the country did not predict how rapid the spread would be and was therefore under-prepared. The very possibility of a second wave was not considered serious enough, and all aspects of the pandemic response bore the brunt, including vaccination. Only an early lockdown could have helped India soften the deadly impact of the second wave—a solution that India could ill-afford then, although most states soon got into lockdowns after the infection spread far and wide. In retrospect, in choosing a slow rollout to a larger population rather than rapid vaccination of specific groups, the policy goal shifted from saving lives in the short run, to controlling infection and disease in the medium term. Delaying the opening up of vaccination to all of the 18-44 age category— and instead targeting the high-risk categories within the group, when supply of sufficient doses could not be ensured—may have saved many lives in the current wave, at least in the second half.
Next Steps
An effective response to the pandemic requires a three-pronged approach: (a) treatment of the affected population; (b) tracking and slowing the spread of the virus; and (c) protection of the rest of the population who are still not exposed to the virus, and therefore, more vulnerable.
This report offers the following recommendations for systematically tackling this crisis.
1. Strategic monthly planning. Both Centre and states must initiate specific monthly schedules for vaccination. This will help in monitoring the progress of vaccination across geographies and evaluating bottlenecks in the vaccine delivery process in a timely manner. Such a system will also make governments responsible for the proper and timely resolution of issues. Complete transparency must be introduced in the allocation to states to ensure greater trust and coordination.
2. Targeted vaccination campaigns and risk communication. Given the vaccine shortages that have been reported across the country, the high-risk populations as well as potential super-spreader groups among the active and mobile populations need to be actively identified and inoculated in an intensified campaign mode. India has a history of having a substantial proportion of the population remaining unaware of even the flagship schemes of the government. Local governments, health workers, and community, political as well as religious leaders will have to be incentivised to help identify and vaccinate the most vulnerable. India should remain vigilant in the enforcement of appropriate social distancing behaviours, preventing large gatherings, increasing testing levels, and tracking the spread of the virus by targeted genome sequencing and sharing of data. This will help prevent future waves.
3. Countering the gender and income divide. There is a national gender gap of four per cent, in favour of males, in India’s COVID-19 vaccination programme. Current data shows that only four states have a higher female-to-male vaccine dose ratio, while states such as Delhi and Uttar Pradesh have a male-female gap of more than 10 per cent. Furthermore, anecdotal evidence suggests that many smaller towns and villages are seeing a shortage of vaccine doses for their domestic population, as people from urban areas have been getting inoculated there, taking advantage of the first-come-first-served principle of COWIN portal’s online registration. Apart from the obvious equity concerns, such gaps in vaccine delivery may allow for mutation of the virus within people who are not vaccinated, possibly leading to the virus becoming immune to the vaccine, and new variants attacking urban areas again. The digital divide should not be allowed to get translated to a vaccine divide.
The government must consider eliminating dual pricing between the Centre and states and keep providing the vaccines free in all government facilities, and for Rs 250 in private hospitals. Under the current circumstances, allocating national resources to the COVID-19 vaccination drive could prove to have the highest return on investment for the Indian economy. In the course of the pandemic, many Central Acts have been invoked, including the Epidemic Disease Act 1987, Epidemic Diseases Amendment Act, 2020 and Disaster Management Act 2005. In the spirit of the key coordinating role of the central government, access to COVID-19 vaccine for all needs to be seen as a Central government responsibility. The onus of vaccine procurement, whether domestic or international, should be with the Central government. States must be allowed sufficient flexibility in their respective distribution strategies.
4. Proactive procurement and expanding manufacturing capacity. In light of the lukewarm response to the global tenders floated by state governments, the Centre should step in and initiate bulk procurement. Amidst a global shortage of vaccines, fragmenting purchasing power may mean that the states end up with outcomes that are both iniquitous and inefficient. Government agencies must start engaging constructively with vaccine developers and producers to anticipate raw material requirements and ensure maintenance of necessary supply lines. This also means fast-tracking development and regulatory approvals with regard to the vaccines currently in different stages of development and trials. Advance orders beyond July should be made, to ensure future supplies, and provide liquidity for the vaccine producers. By the end of 2021 or beginning of 2022, India should enhance its vaccine manufacturing capacity to the extent of addressing the global requirement of vaccines, and for that, drastic measures are required, like reviving large PSU facilities such as HLL Biotech, with a state-of-the-art vaccine complex.
5. Focus on rural regions and planning for new variants. For a country as vast and resource-constrained as India, quick universal vaccination may not be practical. The country indeed must try to pre-empt new variants through quick and comprehensive vaccination rollout, but given the vastness of the geography and the numbers that need to be covered, the system must be ready for pools of infection spawning new variants. As case numbers go down, sequencing of a sufficiently large sample size from sub-district levels should be aimed for, and proactive funding of further research and development of new vaccines to handle new variants should be conducted in parallel, along with strategies for providing booster shots as and when required.
This paper was first published on ORF.
Read all the Latest News, Breaking News and Coronavirus News here.




















Comments
0 comment