views
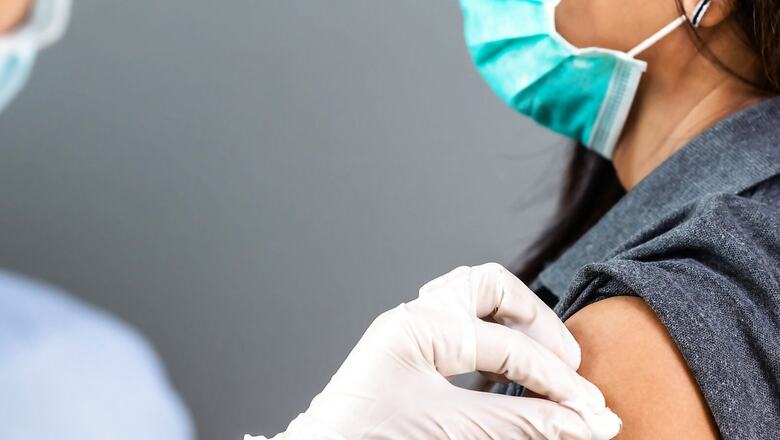
Hyderabad-based Biological E is likely to submit the data for phase 1 and 2 clinical trials by the end of September or early October for emergency use authorisation of its coronavirus vaccine — Corbevax, News18 has learnt. Corbevax may receive approval based on the interim data of phase 1 and phase 2 clinical trials — similar to Bharat Biotech’s Covaxin. The results of phase 3 trials are expected to take around seven more months.
Corbevax, the third made-in-India jab against coronavirus, is an recombinant protein sub-unit vaccine, which is made by using a specific part of the SARS-CoV-2 virus — the spike protein on the virus’s surface. The other two indigenous vaccines are Bharat Biotech’s Covaxin and Zydus Cadila’s ZyCoV-D.
According to two senior government officials, the company has been asked to submit the phase 1 and phase 2 trial data after completing the interim analysis of its efficacy. “The company is engaged in the corroboration of data and interim analysis. As soon as they finish the due process, they will submit the data to the subject expert committee (SEC) and Drug Controller General of India (DCGI),” said a government official privy to the development. “We are hoping to receive the data by the end of September or by early October.”
News18 reached out to Biological E through email for an official comment on the matter, but there was no response till the time of publishing this report.
Vaccine may receive ‘conditional approval’ similar to Covaxin
A second official, meanwhile, said that the vaccine, so far, has proven to be safe. “We need to understand its efficacy. While the platform used for manufacturing the vaccine is highly effective and has been used in multiple vaccines since the last many decades, the due process will be followed before it gets the final approval.”
He further added that if approved on the basis of phase 2 clinical data, the same rules applied to Covaxin (earlier) will be followed. “Covaxin was given conditional approval till the time their phase 3 trials were concluded. The follow up (among vaccine recipients) was more stringent and frequent. However, the final decision (on Corbevax) will be taken after deliberations by the SEC and DCGI,” the second official said, adding that “the company has already started producing a stockpile. The immediate roll-out is possible as soon as it gets approval”.
The central government has placed an advance order for 30 crore doses of Corbevax. It is a two-dose vaccine with an interval of 28 days.
Third phase of clinical trial to roll out soon
On September 1, the DCGI gave permission to the company to conduct phase 2/3 clinical trials of Corbevax on children between five-18 years with certain conditions.
Among adults (18-80 years), the preparations of phase 3 study are in full swing at around 30 sites across India. News18.com spoke to half a dozen investigators at the selected sites who said that the trial is expected to begin in the next 15-20 days.
The targeted sample size is 2,140 subjects and the study duration is approximately seven months. According to the study protocol, the efficacy of the vaccine will be tested against Serum Institute of India’s Covishield, which has been chosen as a comparator for another arm.
Read all minute-by-minute news updates for Uttar Pradesh election results 2022, Punjab election results 2022, Uttarakhand election results 2022, Manipur election results 2022, and Goa election results 2022.
Click here for seat-wise LIVE result updates.
















Comments
0 comment