
views
Electrolyzing Water

Pour water into a glass container. Avoid using plastic or metal for this. You will be running an electric current through the water that could melt plastics. Metal will conduct this current and could shock you if you touch the container. If you are unfamiliar with this experiment, start with one cup of water. Producing too much hydrogen can be dangerous. Children should only do this experiment with a responsible/knowledgeable adult. Add a tablespoon of salt to the water for better results — salt will help conduct the electrical current.
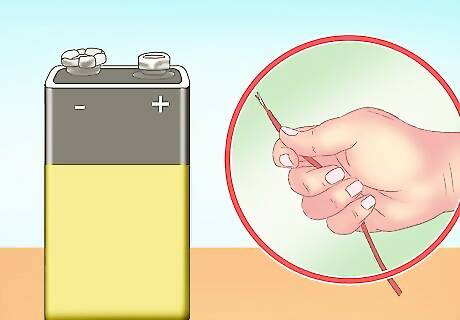
Locate a power source. While large-scale hydrogen production requires a lot of energy, this experiment can be done with little input. A 9V battery is sufficient to perform the electrolysis (splitting of the water into hydrogen and oxygen). You can also use multiple batteries in series to strengthen the effect. A portable power supply is good for this experiment, as this experiment should be done outside or under a fume hood. While batteries aren’t generally dangerous, you should still wear rubber gloves to avoid being shocked.
Connect a paper clip to each terminal of the battery. This will create an anode (the negative paper clip) and a cathode (the positive paper clip). Just wrap the paper clips around the terminals of the battery until they're secure.
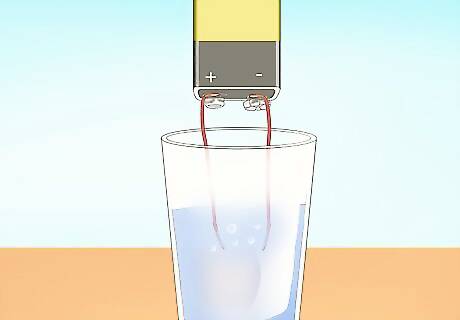
Submerge the paper clips. When you submerge the paper clips, electricity will flow from the anode, through the water, and to the cathode. Hydrogen bubbles will form at the anode and the cathode will produce oxygen and chlorine gas. Be cautious not to touch the paper clips together.
Reacting Acids

Place aluminum foil in a beaker. Tear up pieces of aluminum foil and put them into a beaker or other container. Do not use a container with a lid or cap, as this could result in an explosion. There is no need to measure the amount of aluminum used. You could tear a three inch by three inch square of aluminum foil into pieces about the size of your thumb.

Keep the beaker ventilated. Do this experiment outside or under a fume hood. Hydrogen gas disperses quickly, but is very flammable. A buildup of hydrogen gas that is exposed to the air (or any other source of oxygen) can explode. One example of hydrogen gas exploding is the Hindenburg.

Add hydrochloric acid. There is no need to precisely measure the amount of hydrochloric acid used. The chlorine in the in the hydrochloric acid will react with the aluminum to form aluminum chloride. This will result in the formation of hydrogen gas. Start with about two ounces of hydrochloric acid and add more if needed.
Collecting the Hydrogen

Use a balloon or bottle to collect the hydrogen. Place the opening of the collection vessel (bottle or balloon) over the opening of your reaction beaker or container. The hydrogen gas is less dense than air and will move up into your collection vessel. This method is often used to blow up a balloon to demonstrate the upward movement of hydrogen gas.
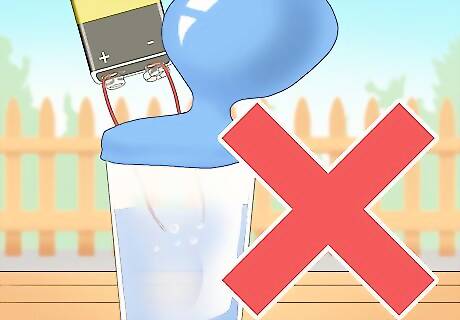
Avoid collecting hydrogen when both of the paper clips are releasing gas. Hydrogen gas can explode if it comes into contact with oxygen. If you are doing electrolysis, do not try to collect any hydrogen when the paper clips are still emitting gas since the gases will be a mixture of hydrogen and oxygen.
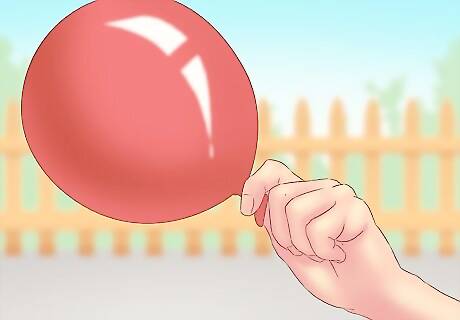
Collect only small amounts. You should not collect or store a high volume of hydrogen gas. It poses a serious safety risk, and is difficult to contain. You should do this experiment only for entertainment or educational purposes.















Comments
0 comment