views
In the thick of allegations of contaminated eye drops sold in the American market by Chennai-based Global Pharma Healthcare, India’s apex pharmaceuticals export council has sought details from the company on the internal investigation within the next two days, News18.com has learnt.
Pharmexcil— an agency that functions under the ministry of commerce and industry — wrote a letter to the managing director of Global Pharma Healthcare, Dr Juma Venkatesh, on February 3. The letter, accessed by News18, mentions that the company has been registered with Pharmexcil since 2005 as a small-scale manufacturer.
As the news has gained the international spotlight, Pharmexcil (in the letter) has told the company that such an incident brings “a bad reputation to the Indian pharma industry and is also likely to have an impact on the trust of international agencies on Indian pharma exports”.
When contacted by News18.com, Udaya Bhaskar, director general of Pharmexcil, said that “the incident is a big blow to the industry”.
“The US is a very important market…not only with respect to 30% of our exports…and the US FDA (Food and Drug Administration) is considered to be one of the more stringent regulatory agencies,” he said.
He further told News18.com that India earned a lot of confidence in its manufactured medicines throughout the world by supplying a huge percentage to the United States and having the highest number of US FDA-approved sites.
Details of the incident
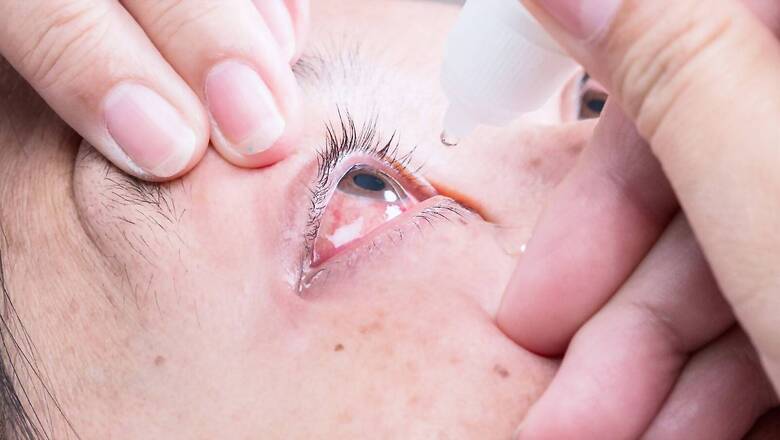
The pharma company is voluntarily recalling all lots of its eye lubricant – EzriCare Artificial Tears – allegedly linked to causing a death in the United States due to possible contamination.
While the American health agency — the US Centers for Disease Control and Prevention (CDC) — is testing unopened bottles of EzriCare Artificial Tears, the United States Food and Drug Administration (FDA) said it has moved to restrict imports of products made by the company.
Global Pharma has suspended the production of a line of eye drops from the United States market.
Pharmexcil seeks info, lashes out at firm
The letter titled “CDC, USA Alert on Artificial Tears manufactured by Global Pharma Healthcare Pvt Ltd — Request for Information”, briefs the entire incident.
“It has come to our notice that The Centers for Disease Control and Prevention (CDC), USA has issued Health Alert Network (HAN) Health Advisory about infections with an extensively drug-resistant strain…in 12 states,” the letter reads.
It mentions that “the news is also being published by several media houses and telecasted all over about the incident and to date, there are 55 reports of adverse events including eye infections, permanent loss of vision, and a death with a bloodstream”.
“It is alleged that the eye drops manufactured and supplied by your company have caused eye infections, permanent loss of vision, and death, bringing a bad reputation to the Indian pharma industry and also likely to have an impact on the trust of International agencies on Indian pharma exports,” the letter written by Bhaskar says.
The agency has asked the company to furnish the details of the licensees to whom it has supplied the subject drugs and manufacturing licence copies and product permissions of subject products.
Bhaskar, in the letter, has advised the company to investigate the reasons for the serious adverse events and update Pharmexcil with the findings within two days from the receipt of the email to enable the agency to inform the ministry (department of commerce) and to take necessary further action.
US a key market for Indian pharma
The United States is the biggest market for pharma exports from India. According to government data, out of the total pharmaceutical exports, 30 per cent share is captured by the American market.
In the financial year 21-22, India exported $7101.60 million (Rs 56,812 crore) worth of products and in the financial year 22-23 (April- December), the exports stood at $ 5630.71 million (Rs 45,045 crore).
On Friday, the central government formed a team of six officers and inspectors to raid the units of the Chennai-based pharmaceutical company.
According to government sources, the teams from the apex health agency, Central Drugs Standard Control Organisation, and officials from the state drug controllers’ office were sent to the plant for further investigations and sample pickups.
The pharma company is a contract manufacturing plant supplying through others to the US market. This specific drug is not sold in India.
Read all the Latest India News here

















Comments
0 comment